Puhtuse teisel poolel:
- 4526
Puhtuse teisel poolel: mida pöördosmoosi membraan suudab ja mida mitte
Pöördosmoosi vesi on igas mõttes H2O / lisandi dihhotoomia näide.
Maailm jaguneb:
1. need, kes usuvad, et osmootne filter puhastab kõike peale karma ja südametunnistuse
2. ja need, kes valavad osmootset vett vaenlase tee sisse, uskudes, et ta on surnud.
Mõlemad eeldused on isikliku veendumuse küsimus. Võtsime endale ülesandeks rääkida, kuidas on lood osmootse veega pärismaailmas ning lisada olemasolevale mustvalgele pildile pastelseid värve.
Räägime membraani tööpõhimõttest, destillaadist ja elektrolüüdist saadava osmootse vee erinevusest ning ka sellest, kas tasub sealt poore otsida ja happes keeta.
Inimese tutvus poolläbilaskvate membraanidega sai alguse tähelepaneliku prantsuse abti Nolet’ loost 18. sajandi keskel. Ta valas veini sea põide ja jättis selle veetünni hoidmiseks. Vein muutus mahla sarnaseks, mull suurenes ja nähtus sai Nolelt nime osmoos (kreekakeelsest sõnast “rõhk”). Abt kirjeldas poolläbilaskva membraani omadusi ja selle peamist võimet läbida ainult vett. Kui veinimull, mille Nole vette pani, ei omaks venivusvõimet, tõstaks läbitungiv vesi rõhku ja protsess peatuks. Survet, mida tuleb rakendada, et vesi ei satuks veini, nimetatakse osmootseks rõhuks. See sõltub lahustunud ainete kontsentratsioonide erinevusest membraani mõlemal küljel.
Kui abt oleks arvanud mulli veiniga välja pigistada ja sellest liigse vee “välja pigistada”, oleks ta võinud samal ajal leiutada pöördosmoosifiltri.
Hiljem liitusid uurimistööga loodusteadlased, botaanikud ja füsioloogid, keda huvitasid osmoosi loomulikud ilmingud, eelkõige taimede ja inimkeha rakkude toitumine. Eraldi huviala moodustasid füüsikud ja keemikud, kes tundsid muret magevee magestamise ja merevee magestamise ülesande "tööstuslikus mastaabis protsessi kordamise" pärast.
Kodumajapidamises kasutatava pöördosmoosi membraani tööpõhimõte
Tänapäeval on pöördosmoosi membraan õhuke polümeerkile, mis on ladestunud inertsele substraadile, mis on vett täielikult läbilaskev. Membraani kõige olulisem omadus on võime paisuda – see tähendab molekulidega reageerida ja nendega seonduda. Seda protsessi nimetatakse hüdratatsiooniks. Teised vees lahustunud ained ei saa membraanimaterjaliga reageerida ning kui paisunud membraanile avaldada veevarustuses olev veesurve, hakkavad läbi membraani imbuma (välja pressima) ainult veemolekulid.
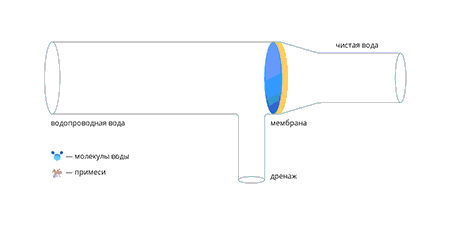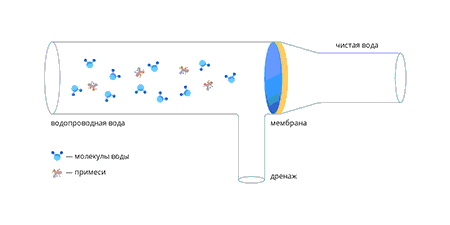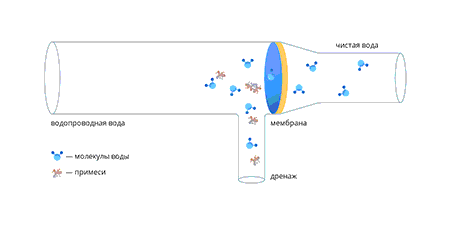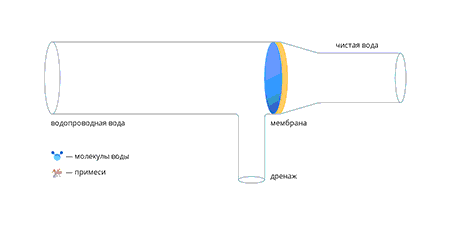
Kui vesi läbib membraani, suureneb lahustunud ainete kontsentratsioon membraani ees ja vastavalt suureneb osmootne rõhk.
Kui osmootne rõhk on võrdne süsteemi rõhuga, peatub vee läbimine membraanist. Et seda ei juhtuks, lastakse kontsentraati pidevalt äravoolu.
Millest on valmistatud kaasaegsed membraanid?
Viimastel aastakümnetel on membraanimaterjale muudetud, millest kõige levinumad, märgime:
- polüatsetaat
Tselluloos. Vana põlvkonna poolläbilaskvad membraanid, mis läbisid kuni 50% nitraate. Süsiniku eelfiltreerimise olemasolu sel juhul ei aita, sest see ei "näe" ka nitraate. Polüatsetaatmembraanide tselluloosi alus kutsus esile bakterite aktiivse paljunemise.
- polüamiid
Viimasel kümnendil on seda tüüpi membraanid laialt levinud eelkõige tänu oma bioidanemiskindlusele ja selektiivsusele 92–99%. AQUAPHOR kasutab oma pöördosmoosisüsteemides polüamiidi 66, mis on sisuliselt nailon.
Eristada tuleks majapidamises kasutatavaid õhukese kilega membraane ja merevee magestamise membraane. Nende membraanide tööpõhimõte on sama, kuid tehniliselt on magestamise membraan erinevalt paigutatud. Mereveest H2O “välja pigistamiseks” on vaja ületada selle kõrgem osmootne rõhk, õhukese kilega membraan puruneb sellistes tingimustes. Suure koormusega töötamiseks magestamise ajal on vaja teistsugust tehnilist konstruktsiooni: membraan on valmistatud muudest materjalidest ja sellel on tihedam substraat (näiteks keraamiline).
Osmoos ei ole sõel!
Arvamus, et membraan töötab "väga väikeste pooride" olemasolu tõttu, ei vasta tõele. Pöördosmoosi membraanil pole poore. Vee eraldumine permeaadiks (puhastatud veeks) ja retentaadiks (kanalisatsiooni suunduvate lisandite kontsentraat) toimub protsessi tõttu, mis sarnaneb elektrivoolu ülekandega läbi metallist pooljuhi.
Võime "juhtida" vett on teatud klassi polümeersete materjalide omadus, mis sarnaneb metallide võimega juhtida elektrivoolu. Samas on materjale, mis ei juhi ei üht ega teist.
Veemolekulide membraani kaudu ülekandmise mehhanism on sarnane vooluülekande protsessiga läbi metalljuhi. Selles pole auke, nagu ka membraanis, sellegipoolest järgneb vool elektronide kujul läbi materjali kohast, kus neid on palju, madalama "kontsentratsiooni" suunas.
Miks ei ole membraani selektiivsus alati 100%?
Võrdleme sorptsiooni- ja pöördosmoosfiltrite filtreerimisvõimet saasteliikide lõikes:
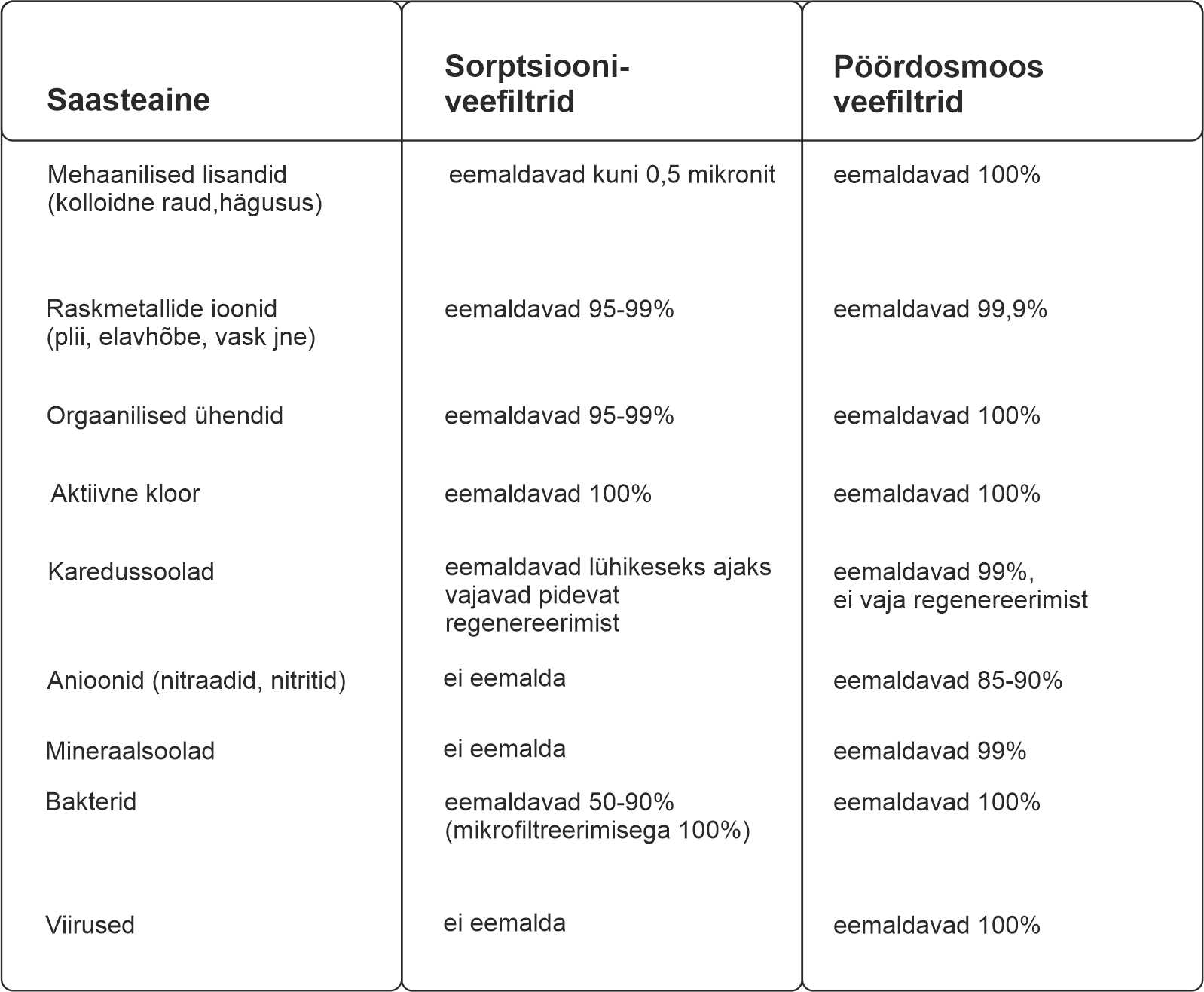
Kõiki lisandeid ei saa 100% eemaldada isegi pöördosmoosi membraaniga. Tuletage meelde, et algselt loodi membraanid vee magestamise jaoks (piirkondades, kus joogivesi on märgatavalt soolane, kuid mitte veel meri). Seetõttu viidi keedusoola (naatriumkloriid) lahusega läbi standardkatsed soolade eemaldamiseks membraaniga. Tõepoolest, osmoos võib saavutada 99% soola eemaldamise. Kui vesi on aga väga kare, võib "läbimurde" suurenemise tõttu kasutegur langeda 93-95%-ni.
Koduse osmoosi jaoks kasutatakse kõige sagedamini membraane, mille selektiivsus on 97–99%. Need on naatriumkloriidi jaoks normaliseeritud, kuid see ei tähenda, et see oleks sama ka teiste ainete puhul. Erinevate saasteainete puhul võib “läbimurre” olla erinev, see sõltub nende olemusest. Näiteks mõned booriühendid läbivad membraani üsna edukalt, teised ühendid, näiteks suured orgaanilised molekulid, vastupidi, eemaldatakse peaaegu 100%.
"libisemine" toimub kolmel põhjusel:
1. "Mimikri". Kui vees on midagi, mille keemiline käitumine on sarnane veemolekuliga, võib see moodustada sidemeid membraanimaterjaliga ja "mööda minna".
2. Difusioon, sellest räägime hiljem täpsemalt.
3. Membraani kahjustus või halb kvaliteet.
Mimikri kohta. Kujutage ette rida töötajaid, kes liiguvad telliseid ketti mööda. Kui mitu tellist asendada millegi väga sarnase ehk "raske ja ristkülikukujulise eseme" vastu, on ebatõenäoline, et keegi ahelas olijast asendust märkaks.
Igasugune membraan laseb läbi teatud koguse lahustunud aineid, mistõttu TDS-mõõturiga soolsuse (ja tegelikult ka elektrijuhtivuse) mõõtmised näitavad väga madalaid tulemusi, kuid mitte nulli.
Difusioon on paralleelne protsess
* Difusioon on mittetasakaaluline protsess, mille käigus aine viiakse kõrge kontsentratsiooniga piirkonnast madala kontsentratsiooniga piirkonda,
mis viib kontsentratsioonide spontaanse ühtlustumiseni kogu hõivatud ruumala ulatuses.
Samaaegselt veemolekulide membraani läbimise protsessiga toimub ka lahustunud ainete difusiooniprotsess läbi selle. Mida suurem on kontsentratsioonigradient, seda suurem on difusioon. Loomulikult määrab selle protsessi tulemuse ka hajuvate ainete iseloom: mõni neist on rohkem "närvikas", mõni vähem. Kui muud asjaolud on võrdsed, difundeeruvad suured orgaanilised ioonid halvemini kui väikesed, "nõrgad" leelismetalliioonid.
Võrreldes veemolekulide peamise transpordiga läbi membraani, on difundeeruva aine kogus väike ja selle võib majapidamisveepuhastusel tähelepanuta jätta. Kuid just sel põhjusel ei ole membraani selektiivsus 100%.
Difusiooni tulemus on tavaliselt märgatav esimeses veekogus pärast pikka seismist – filtri seisakuid. Selle aja jooksul on soolade kontsentratsioon membraani mõlemal küljel aega ühtlustada. "Täiustatud" filtritel on selle probleemiga toimetulemiseks spetsiaalsed nipid.
Mis tahes materjal allub difusioonile. Kas polüetüleen on teie arvates õhukindel? Gaasid läbivad seda vilega, kuigi vaikselt. Heelium hajub õhupallist palju kiiremini.
Mida ei puhasta isegi pöördosmoosfilter?
On aineid, mis membraani kergesti petavad. Nende hulgas on boor/boraadid. Neutraalse pH juures on boor lahuses H3BO3 molekulina ja on mõne omaduse poolest väga sarnane membraanis oleva veega. See võimaldab booril veega koos membraani läbida. Kui pH muudetakse leeliseliseks, on boor lahuses laetud ioonina - boorhappe või tetraboraadi anioonina. Anioonina on boor membraaniga juba täiuslikult ära lõigatud.
Mõnda väga lenduvat orgaanilist ühendit iseloomustab kõrge difusiooniaktiivsus. Näiteks kloroform suudab läbi membraani tungida, kuid süsinikeelfiltri abil on see kergesti eemaldatav. Membraan ei ole mõeldud gaaside, eriti vesiniksulfiidi eemaldamiseks. Megalinnade elanikud ei peaks muretsema, vesiniksulfiidiga vett ei panda veevarustusvõrku ja boor pole igal kujul mürgine. Boorhapet tilgutatakse näiteks lastele kõrva.
Membraani "tervise" tegurid
Põhjused, miks membraan ebaõnnestub:
1. Füüsiline kahju
2. Hüdratsioonivõime kaotus klooritud vee või muude oksüdeerivate ainetega kokkupuutel (osoonimine) või membraani kuivamise tõttu. Protsess võib muutuda pöördumatuks, seetõttu ei tohiks lubada juba töödeldud membraani kuivatamist. Ja selleks, et vältida membraani kahjustamist kloori tõttu, sisaldab pöördosmoosi filter tingimata süsiniku eelfiltreid.
3. Vees olevate lahustumatute soolade ja mehaaniliste lisandite sadestumine pinnale (halb / ebapiisav eelfiltratsioon)
4. Ebapiisav veevool äravoolu või äravooluvee “kokkuhoid”.
Parim - hea vaenlane?
Paradoksaalsel kombel võivad paljud puuduseks pidada võimet peaaegu täielikult puhastada vett lisanditest. Miinusena kajastatakse ka drenaaživee filtreerimise põhimõtet "tegevuskulude nõelale" istutamise egiidi all. Oleme koostanud nende ja sarnaste küsimuste kohta väikese KKK.
1. Kas vesi on "surnud"? (meie lemmik)
Mida mõiste "surnud vesi" armastajad mõtlevad, pole meile täiesti selge. Ametliku teaduse seisukohalt pole ei elavat ega surnud vett. Peaaegu iga H2O molekul planeedil on kunagi olnud vihmapiisas ja mõne organismi jääkproduktides. Vee vapustavaid omadusi pole – looduses on selle ringlemine, aga ka suurepärane võimalus membraaniga vesilahust kogu alluviaalist puhastada. Teeme ettepaneku käsitleda muinasjutte ainult kultuuriressursina, kuna meie keha vajab H2O-d, ülejäänud jagunevad kahte rühma:
1. valikuline, kuna see on toiduga kaasas;
2. lühemas või pikemas perspektiivis ebatervislik.
Lisandite puudumine joogivees ja koos nendega nn "kasulikud mineraalid" ei talu toitumiskriitikat. Kaltsium meie kehas veest praktiliselt ei imendu, kuna seda leidub seal anorgaaniliste soolade kujul. Isegi kui see kaltsium imenduks, oleks raske juua 17 liitrit keskmise karedusega Moskva vett, et rahuldada selle elemendi päevane vajadus - umbes 1000 mg. Veest saab hästi imenduda ainult magneesium, aga sellest rääkisime eraldi.
2. Kas selle ülalpidamine on kallis?
Arvestades 1/6 kaotust äravoolule, saab kergesti välja arvutada tohutu erinevuse kodus puhta vee hankimise ja poest joogivee lõpphinna vahel ning plastikus oleva vee kvaliteet on sarnane, kui mitte halvem.
3. Miks ei ole pöördosmoosi vesi destillaat?
Vesi ei ole meie jaoks toit ja mitte viis saada "telliseid" keha ehitamiseks. See on keskkond, kus toimuvad keha keemilised ja füüsikalised protsessid. Pealegi on ta ise üsna inertne ega osale nendes protsessides peaaegu kunagi. Me ei jaga seda vesinikuks ja hapnikuks, kehas elektrolüüsiprotsess ei toimu.
Mõistes seda vee rolli, võite juua ka destilleeritud vett, mis ei sisalda "kasulikke mineraale". Tasakaalustatud toitumisega ei teki teil probleeme.
Destilleeritud vee oht on see, et see võib olla lihtsalt "määrdunud". Aurustumine ei vabasta vett orgaaniliste ainete lisanditest, mille keemistemperatuur on alla 100C.
Pöördosmoosi membraanijärgse vee ja destilleeritud vee vahel on tohutu erinevus. Pöördosmoos ei lõika lahustunud sooli täielikult ära, kuid destilleerimisel jäävad soolad täielikult destilleerimiskuubi sisse. Teisest küljest liiguvad destilleerimisprotsessi käigus lenduvad orgaanilised ained koos auruga destillaati, samas kui membraan eemaldab need hästi. Lisaks olid varasematel destilleerijatel kummist torud, mis lisasid tekkinud veele "maitsetust".
4. Miks pöördosmoosi vesi ei ole elektrolüüt?
Jagame oma leidu:
"Osmoosiga puhastatud vett võiks võrrelda destilleeritud veega, kui mitte üks väike,
kuid oluline "aga": puhastamise tulemusena muutub vesi paratamatult kergelt happeliseks, kuna leeliseliste soolade molekulid
ja leelismuldmetallid ja nende koostises olevad ioonid läbivad filtri vabalt.
Selle tulemusena joome iga päev väga-väga nõrga...elektrolüüti."
Elektrolüüt on igasugune vedelik, mis juhib elektrit. Näiteks supp või kompott. See tähendab kõike vedelikku, mis juhib ioonide liikumise tõttu elektrivoolu.
Elektrolüüdid on maitsev lõunasöök.
5. Kas membraan vajab loputamist?
Membraanide läbipesu on tõepoolest tehtud. See kehtib aga tööstuslike membraanide kohta. Nende pesemiseks kasutatakse olenevalt sellest, millised osakesed membraanile "kleepuvad", terve arsenali spetsiaalseid ühendeid: aluselisi, happelisi pesuaineid, pindaktiivseid aineid jne. Tööstuslike membraanide puhul on nende osakeste kohta kõik teada ja koostis valitakse individuaalselt.
Youtube’ist videote vaatamine, kuidas sidrunhappes membraane keedetakse, on veidi kurb, sest meie silme all raiskavad inimesed asjata aega - membraan kaotab kõrgete temperatuuride tõttu oma omadused.

Mida me öelda tahtsime?
1. Ajalooliselt on pöördosmoosi membraanid head, kuna need suudavad suurepäraselt vett magestada, selleks need loodi. See omadus leevendab kannatusi neil, kelle joogivesi on kas väga kare või lihtsalt soolane.
2. Majapidamismembraanid suudavad maksimaalselt välja filtreerida kahjulikke lisandeid, millega tänapäevane maailm vett rikastab. See membraanide võime ületab sorptsioonifiltrite võimed. Ioonivahetuskassettide puhul võivad sorptsioonifiltrid ka kõvadust vähendada. Siiski on tõhususe ja ressursside osas mitmeid tüütuid piiranguid.
3. Pärast osmootset filtrit pole vett kahjustada. Mida puhtam on vesi, seda kergemad on ainevahetusprotsessid organismis.
4. Membraaniga puhta vee liitri maksumus on veidi kõrgem kui sorptsiooniveepuhasti oma. Kuid peate võrdlema puhastusastet poe pudelitega, kuna plastis olevat vett puhastatakse sama tehnoloogia abil.
Kas pöördosmoosi membraani kasutada või mitte, sõltub motivatsioonist ja soovist minimeerida negatiivsete keskkonnategurite mõju. Puudub kasulik vesi, nagu ka kasulik õhk. Kasulik õhk on sissehingamine, kuid see pole enam õhk. Ja meie labori puhta vee valem kõlab järgmiselt:
Vett ei tohiks tee sees näha.

